Triterpenes
Triterpenes as well as mono-, sesqui-, di-, sester- a and tetra- are part of extensive group of natural compounds - terpenoids, which are wide spread not only in plants but in few cases in marine organisms, moulds, microorganisms or rarely in fungi.1,2 Terpenoids with steroids constitute very wide area of isoprenoids. Basic building block of all isoprenoids is isoprene (2-methylbuta-1,3-diene). Biological precursor of triterpenes is squalene. Monoterpenes (two building blocks) - eg. camphor, borneol, menthol, pinene, limonene, carvone, citroneol, iasmon, terpinene, terpineole, geraniol and in part also sesquiterpenes (3 building blocks) - eg. ionones, methylionones, irone, nerolidole, farnesole, cedrole, caryophylen are natural fragrat principles, 3 they constitute major part of etheric oils and resins. Sester-, tetra- and triterpenes on the other hand are mostly solids in natures either free or as esters or glycosides.
Until now more than 30 000 terpenoids was isolated from which there are 4000 triterpenoids derived from more than 40 skeletons 1,2,4. Range of biological activities of triterpenoids is incredibly broad eg. antimicrobial (fusidic and helvolic acid), antifeedant (radermasinin), antimycotic (trianthenol), antiviral (derivatives of betulinic acid), antiinflamatory (derivatives of bosswelic acid, platycodin D, betulin, lupeol), antutumor effects (betulinic, bosswelic, pomolic acid), anticariogenic (oleanolic, glycyrrhetic acid), antiangiogenic, antiulcerogenic (glycyrrhetic, ursolic and oleanolic acid), antialergic (oleanolic acid), hepatoprotective (lupeol, ursolic, oleanolic acid), tonizing activity (24-hydroxytormentic and bosswelic acid) and many other.
Some of the before mentioned are well known for long time from their usage in the oriental or folk medicine5 (for example bark of Brazilian tree Ocotea suaveolens is used in ingenious medicine for pain relief, as tonicum and for asthma treatment)6, so their pharmaceutic potential is used from very small part.
 Betulin is natural triterpenic alcohol (lup-20(29)-en-3β,28-diol), which is widespread in nature, mainly in the birch bark (Betula sp.)7, from which comes its name. Betuline was one of the first triterpenoids to be isolated from natural source by Löwitz in 1788 by sublimation from birch bark as pure chemical substance.7Then it was obtained by extraction of the outer layers of birch bark by ethanol. I took several decades before its structure could be determined. White colour of birch bark is mainly caused by betulin, which gives birch bark specific properties. For example birch bark never rot or mould which is caused by the anti-mould and antibacterial properties of betulin. Big question is why birch synthesises betulin and deposit it into its bark. Probably it is natural agent against pests and microorganisms. Betulin and its derivatives however posses other biological effects, eg. antiinflamatory, antiviral, anti-HIV, hepatoprotective and others.4
Betulin is natural triterpenic alcohol (lup-20(29)-en-3β,28-diol), which is widespread in nature, mainly in the birch bark (Betula sp.)7, from which comes its name. Betuline was one of the first triterpenoids to be isolated from natural source by Löwitz in 1788 by sublimation from birch bark as pure chemical substance.7Then it was obtained by extraction of the outer layers of birch bark by ethanol. I took several decades before its structure could be determined. White colour of birch bark is mainly caused by betulin, which gives birch bark specific properties. For example birch bark never rot or mould which is caused by the anti-mould and antibacterial properties of betulin. Big question is why birch synthesises betulin and deposit it into its bark. Probably it is natural agent against pests and microorganisms. Betulin and its derivatives however posses other biological effects, eg. antiinflamatory, antiviral, anti-HIV, hepatoprotective and others.4
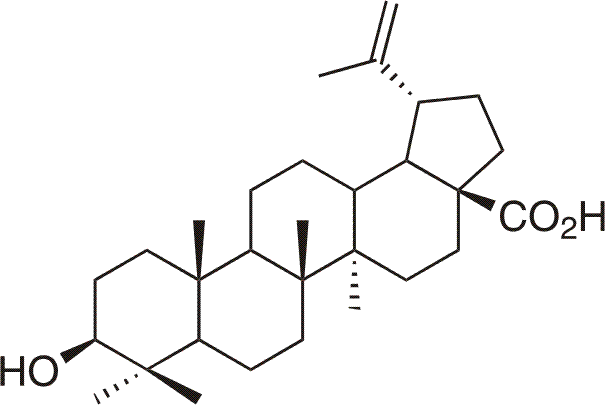 Betulinic acid is natural pentacyclic acid (3β-hydroxylup-20(29)-en-28-oic acid), also widespread in nature. First isolated from Vauquelinia corymbosa,8 and also from african bush Ziziphus mauritiana Lam. (Rhamnaceae)9, also present in Plate bark (Platanus hispanica)10. Betulinic acid was studied in more detail than betulin, mainly due to its selective antitumor activity against human melanoma cell culture. Some of derivatives of betulinic acid also show high anti-HIV and antiviral activities.4
Betulinic acid is natural pentacyclic acid (3β-hydroxylup-20(29)-en-28-oic acid), also widespread in nature. First isolated from Vauquelinia corymbosa,8 and also from african bush Ziziphus mauritiana Lam. (Rhamnaceae)9, also present in Plate bark (Platanus hispanica)10. Betulinic acid was studied in more detail than betulin, mainly due to its selective antitumor activity against human melanoma cell culture. Some of derivatives of betulinic acid also show high anti-HIV and antiviral activities.4
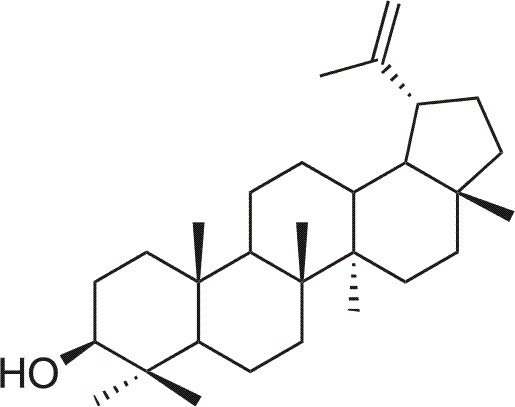 Lupeol is natural pentacyclic triterpenic alcohol (lup-20(29)-en-3β-ol), present in the number of herbs4 or fruit (eg. apples, mango), and also accompanying betulin. Lupeol shows some chemopreventive, cytotoxic and antifeedant properties 4, and also direct antioxidant effects and is potential drug againts free radical inflicted diseases4. Among other activities are antiangiogenic effect (from 30 µg/ml)4.
Lupeol is natural pentacyclic triterpenic alcohol (lup-20(29)-en-3β-ol), present in the number of herbs4 or fruit (eg. apples, mango), and also accompanying betulin. Lupeol shows some chemopreventive, cytotoxic and antifeedant properties 4, and also direct antioxidant effects and is potential drug againts free radical inflicted diseases4. Among other activities are antiangiogenic effect (from 30 µg/ml)4.
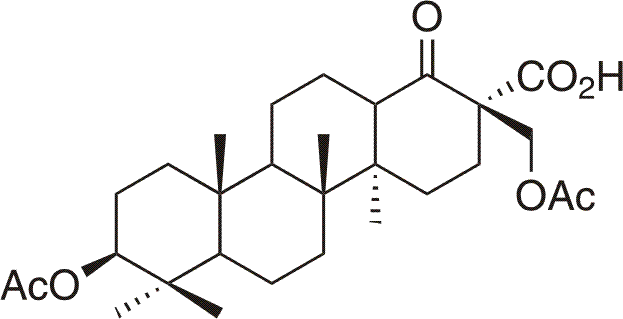
Betulinines11 represents group of triterpenoids prepared by Jan Sarek in 1999-2001 in the Natural compounds research group, Department of organic chemistry, Faculty of science, Charles university. The compounds, derived from lupane, des-E-lupane and 18alpha-oleanane skeletons, posses, between many others, significant cytotoxic activity (IC50 < 5 µmol/l). In contrast to longest known cytotoxic triterpenoid - betulinic acid - antitumor activity is not restricted to cell lines of melanoma (meL-2). Betulinines posses broad range of antitumor activity against many cancer cell lines of different histogenetic origin including multidrug resistant11, eg. A549, Saos2, BT549, DU145, OVCAR-3, K562, U2OS, CEM and so on. This effectivity reaches as far as p53 negative cells, pRb negative cells regardless of their histogenetic origin, with low toxicity against non-cancer cells.11 From the morphologic analysis tissue cell culture with most effective compound - betaketoacid(3β,28-diacetoxy-19,20,21,29,30-pentanorlupan-22-oic acid), which show on the most cell lines cytotoxicity lower than 1 µmol/l, arose conclusion that mechanism of action is apoptosis (programmed cell death), see next picture.11

Flow cytometry studies shown that betaketoacid induce very fast apoptosis, even faster than with paclitaxel, one of the most effective conventional cytostatics. According to current knowledge apoptosis is induced by activation of caspase cascade by release of cytochrome c or AIF (apoptosis inducing factor). Betaketoacid is causing block in G1/S phase of cell cycle. According to latest experiments this compound posses unique ability to block last step of respiratory chain and subsequently start production of peroxide radical (see picture). Blocking is probably caused due to direct conjugation of betaketoacid with cytochrome c. Primary molecular target is therefore localised in mitochondria.12

References:
1. J. D. Connolly and R. A. Hill: Nat. Prod. Rep. 22, 230 (2005).2. J. D. Connolly and R. A. Hill, Nat. Prod. Rep. 22, 487 (2005).
3. Vonášek F., Trepková E., Novotný L.: Látky vonné a chuťové, SNTL, Praha 1987.
4. Dzubak P., Hajduch M., Vydra D., Hustova A., Kvasnica M., Biedermann D., Markova L., Urban M., Sarek J.: Nat. Prod. Rep. 23, 394 (2006).
5. Kosuge T, Yokota M, Sugiyama K, Mure T, Yamazawa H, Yamamoto T.: Chem. Pharm. Bull. 33, 5355 (1985).
6. A.Beirith,A.R.Santos,J.B.Calixto,S.C.Hess,I.Messana,F. Ferrari and R.A.Yunes, Planta Med. 65, 50 (1999).
7. Simonsen J., Ross W. C. J.: The Terpenes IV, Cambridge Univ. Press, London 1957.
8. Trumbull E. R., Bianchi E., Eckert D. J., Wiedhopf R. M., Cole J. R.: Pharm. Sci. 65, 1407 (1976).
9. Simonsen J., Ross W. C. J.: The Terpenes. V, Cambridge Univ. Press, London 1957.
10. Urban M., Sarek J., Klinot J., Korinkova G., Hajduch M.: J. Nat. Prod. 67, 1100 (2004).
11. Sarek J., Klinot J., Dzubak P., Klinotova E., Noskova V., Krecek V., Korinkova G., Thomson J. O., Janostakova A., Wang S., Parsons S., Fischer P. M., Zhelev N. Z., Hajduch M.: J. Med. Chem. 46, 5402 (2003).
12. Dzubak P., Sarek J., Anzenbacher P., Masek V., Novak P., Havlicek V., Vydra D., and Hajduch M.: Cancer Res., submit, 2008.
